
Please click on the agreement button below to acknowledge that you are a healthcare provider.

Atorvaliq®, the first and only FDA-approved Liquid Oral Suspension of atorvastatin calcium for patients 10 years of age and older. Continue scrolling to learn more.
Available in: 150 mL bottle
Dysphagia can include difficulty starting a swallow (oropharyngeal dysphagia), issues in the throat (pharyngeal dysphagia), and the sensation of food being stuck (esophageal dysphagia).
Unfortunately, liquids derived from crushed/compounded tablets raise concerns about patient safety and efficacy, and they have come under increasing scrutiny from the FDA.
It’s important to optimize treatment plans for patients with dysphagia given these complications. Dysphagic patients who suffer from chronic heart failure, edema caused by heart or liver failure, and/or hypertension are often prescribed medications that are crushed/compounded from tablets into a liquid form.
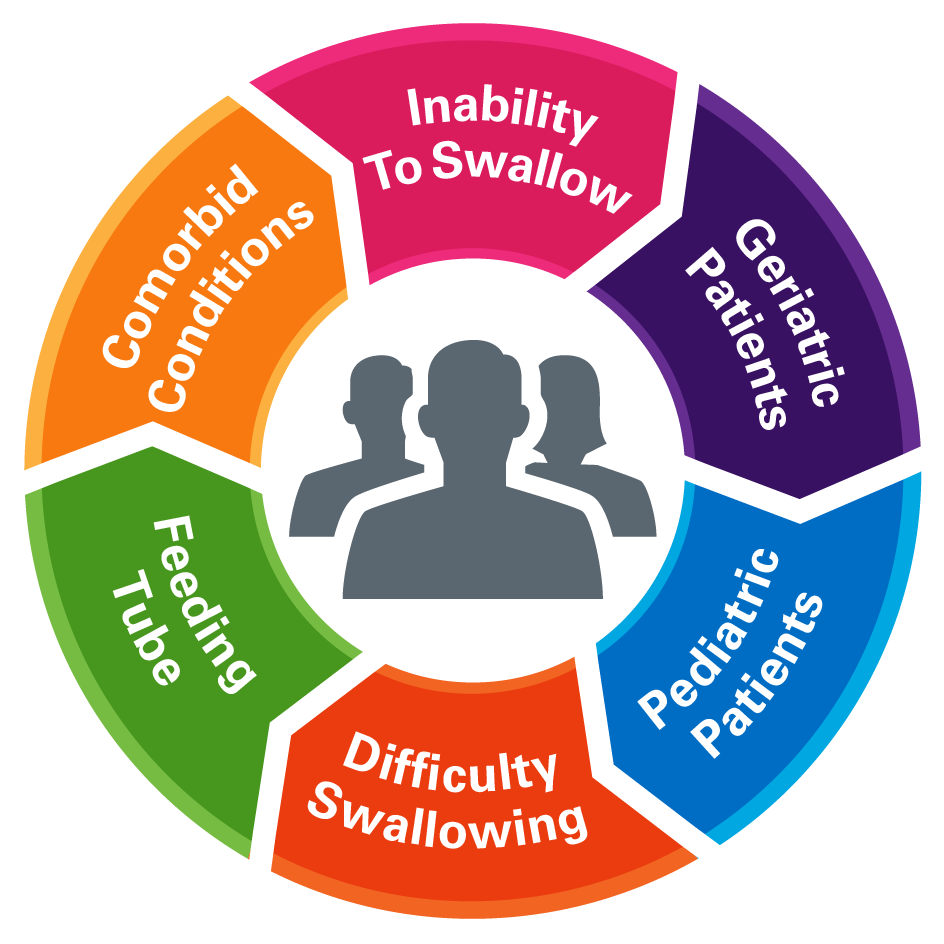
Dysphagic Patients:
Patients who need a liquid formulation may
also have a comorbid condition that
creates difficulty swallowing.
“Compounded drugs should only be distributed to meet the needs of patients whose medical needs cannot be met by an FDA-approved drug”

The range of potency compounded products exhibited in a 2006 FDA survey was 68% to 268%.2 Patients who have difficulty swallowing are often given crushed/compounded formulations of the prescriptions. However, crushed/compounded formulations can exhibit a wide variation in potency due to non-uniformity of compounded materials. These dosing inconsistencies of compounded suspensions have long been a persistent challenge for pharmacists and patients.3
In 2007, the CDC found compounded drugs have a higher risk of contamination.2 Due to fatalities related to contamination, the FDA recently released stricter guidance regarding crushing/compounding and recommended against using crushed/compounded products considered “essentially copies of a commercially available drug product” without permission, especially if an FDA-approved alternative exists.4
Crushing medications can require expensive safety protocols that take up valuable staff time and can trigger additional requirements and guidelines:
• Not following CMS guidelines for crushing medications can result in F-tag violations.1
• Ensuring crushed medications are NOT combined and given all at once either orally or via feeding tube.1
Crushed and compounded products can have variable and very costly, short shelf lives.
1 Milligan, Lynn. “F-Tag 760: Avoiding a Citation When Crushing Medication.” American Association of Post-Acute Care Nursing. Accessed March 2, 2023. https://www.aapacn.org/dns/f-tag-760-avoiding-a-citation-when-crushing-medication/
2 Gudeman, Jennifer, Michael Jozwiakowski, John Chollet, and Michael Randell. “Potential Risks of Pharmacy Compounding.” Drugs in R&D 13, no. 1 (2013): 1-8. doi:10.1007/s40268-013-0005-9.
3 Kindy K, Sun L, Crites A. Compounding pharmacies have been linked to deaths, illnesses for years. Washington Post. February 7, 2013. http://www.washingtonpost.com. Accessed October 2, 2017.
4 Food Drug Administration Center for Drug Evaluation & Research (2016). Guidance for Industry: Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act (FDA, Maryland) 1-8. doi:10.1007/s40268-013-0005-9.
Terms and Conditions
* Void where prohibited by law. CMP Pharma reserves the right to rescind, revoke or amend this program without notice. Offer not valid for patients eligible for benefits under Medicaid (including Medicaid managed care), Medicare, TRICARE, Veterans Affairs, FEHBP, or similar state or federal programs. Offer void where prohibited, taxed, or otherwise restricted. Offer good only in the U.S.A. No generic substitution with this offer.
ATORVALIQ is indicated:
Most common adverse reactions (incidence ≥ 5%) are nasopharyngitis, arthralgia, diarrhea, pain in the extremity, and urinary tract infection.
To report SUSPECTED ADVERSE REACTIONS, contact CMP Pharma, Inc. at 1-844-321-1443 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See full prescribing information for ATORVALIQ dosage modifications due to drug interactions.
Please see full prescribing information for additional safety information.